Article Word Template (Word File)
Conflict of Interest Disclosure Form
Author's Guide
It is necessary to upload these files separately:
1- Main File; without title page (*)
2- Title Page (*)
3- Cover Letter
4- Graphical Abstract (JPEG or cdx)
------------------------------------------------------------
Important note:
- All fields should be completed by authors
- Article similarity (plagiarism) should be less than 15%
- All references should be written based on the journal guide and also with the EndNote software (the EndNote file of the journal is prepared and can be downloaded from the journal site)
- Articles should be in the form of a A4 (Width equal to 21 cm and height equal to 29.7 cm), with a margin of 2.5 cm on all four sides
- Although the main text of journal articles is two columns, authors must submit their articles to the journal from one column. If the article is accepted, at the time of publication, the original text of the article will be in two columns by the journal page designer.
Cell. Mol. Biomed. Rep.: Instructions for Authors
1. General
1.1 Conditions of acceptance
Submission of a manuscript implies that the work has not been published and is not submitted for publication anywhere else. All authors must approve publication. Authors should accept publication fees.
1.2 Conflict of interest
Financial, personal or other conflicts should be disclosed by authors.
1.3 Ethics for animal experiments and medical studies
Experiments should be carried out in accordance with the guidelines laid down by the International animal ethics committee or institutional ethics committee and in accordance with local laws and regulations. Authors are requested to indicate ethical declarations in the Experimental section.
1.4 Open access
All articles published by Cell. Mol. Biomed. Rep. are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
2. Types of papers
Letters in Bioscience publishes Research articles, Short communications, Reviews and others
3. Presentation of manuscripts
Use Camberia 10 with 1.5 line spacing throughout the manuscript. Tables and figures should not be included in main manuscript. Italics should be used in the text for all scientific names and other terms such as genes, mutations, genotypes and alleles. SI units should be used throughout the manuscript.
3.1 Article structure
Manuscripts should be prepared according to the following order
- Title
- Abstract and 5–7 keywords
- Introduction
- Material and Methods
- Results
- Tables (up to 5)
- Figures (up to 5)
- Figure Legends
- Discussion
- Conclusions
- Acknowledgements
- References (up to 35)
3.2 Title page
Title: Concise and informative, avoid abbreviations and formulas where possible
Author names and affiliations: List of all authors with full given and family names (not capitalized), addresses of all authors, name of corresponding author with e-mail address.
3.3 Abstract
The abstract should be concise and factual written in a single paragraph. It should state briefly the purpose of research the principal results and major conclusions. References and abbreviations should be avoided but if essential they must be defined at their first mention in the abstract itself.
Keywords: A maximum of 5 to 7 specific keywords is required
3.4 Introduction
State the objectives of the work and provide an adequate background, avoiding a detailed literature survey or a summary of the results.
3.5 Materials and Methods
Materials and methods section should include the design of the study, the type of materials involved, a clear description of all comparisons, and should be concise but sufficient for repetition by other qualified investigators. Methods already published should be indicated by a reference: only relevant modifications should be described.
3.6 Results
Results should be clear and concise.
3.6.1 Tables:
Authors should use tables only to achieve concise presentation or where the information cannot be given satisfactorily in other ways. Tables should be numbered consecutively using Arabic numerals and should be in the text itself at appropriate place not at the end or as separate attachment. Each table should have an explanatory caption which should be as concise as possible.
3.6.2 Figures:
Authors may use line diagrams and photographs to illustrate these from their text. The figures should be clear, easy to read and of good quality. Styles and fonts should match those in the main body of the article. Lettering and lines should be of uniform density and the lines unbroken. Axis labels should be in bold face. Units should be placed next to variables in parentheses. All figures must be in the text itself appropriate place not at the end or as separate attachment.
3.7 Discussion
This should explore the significance of the results of the work, not repeat them. A combined Results and Discussion section is often appropriate. Avoid extensive citations and discussion of published literature.
3.8 Conclusions
The main conclusions of the study should be presented in under this section.
3.9 Acknowledgements
All contributors who do not meet the criteria for authorship as defined above should be listed in an acknowledgements section. Examples of those who might be acknowledged include a person who provided purely technical help, writing assistance, or a department chair that provided only general support.
3.10 References
The references section must include all relevant published works, and all listed references.
At the end, you will see the writing format in detail
Type of Manuscript: Original Article
Title of Manuscript: (Font; Cambria 16 Bold)
Abstract: (Font; Cambria 10 Bold)
Background: (Font; Cambria 10 Bold)
Text for this section of the abstract (Font; Cambria 10 Regular)
Methods: (Font; Cambria 10 Bold)
Text for this section of the abstract…
Results; (Font; Cambria 10 Bold)
Text for this section of the abstract…
Conclusions: (Font; Cambria 10 Bold)
Text for this section of the abstract…
Keywords: 3 to 6 keywords to describe your manuscripts subject, Taken from the Mesh and PubMed web site and Sort alphabetically.
Write the MeSH Unique ID or PubMed Number (or PMID) of each word in front of it.
https://www.ncbi.nlm.nih.gov/mesh/
https://pubmed.ncbi.nlm.nih.gov/
Main Text:
*Before text of first line of all major paragraphs should be dented by half a centimetre
All main and sub-titles of the main text of the article should be numbered.
1. Background (Font; Cambria 10 Bold)
Text for this section (Font; Cambria 10 Regular)
2. Material and Methods (Font; Cambria 10 Bold)
2.1. Sub- heading for this section (Font; Cambria 10 Bold)
Text for this sub-section. (Font; Cambria 10 Regular)
2.2. Sub- heading for this section (Font; Cambria 10, Bold)
Text for this sub-section. (Font; Cambria 10 Regular)
2.3. Sub- heading for this section (Font; Cambria 10 Bold)
Text for this sub-section. (Font; Cambria 10 Regular)
3. Results (Font; Cambria 10 Bold)
3.1. Sub- heading for this section (Font; Cambria 10 Bold)
Text for this sub-section. (Font; Cambria 10 Regular)
3.2. Sub- heading for this section (Font; Cambria 10 Bold)
Text for this sub-section. (Font; Cambria 10 Regular)
3.3. Sub- heading for this section (Font; Cambria 10 Bold)
Text for this sub-section. (Font; Cambria 10 Regular)
Figures
All Figures/images should be inserted within the text as close as possible to where they are referenced (fig. 1). Authors should not upload them as separate files.
Fig. 1. The leaf and flower of Tecomella undulate (Font; Cambria 10)(reference)

Picture resolution= 300 dpi
*The bottom titles of the figures should be in the middle
Notice: if your pictures are not based on your research you have to cite the reference that you used
Note: The number of figures in each article should not exceed 5
Tables
All Tables should be inserted within the text as close as possible to where they are referenced (table 1). Authors should not upload them as separate files.
Table 2. Effect of temperature on pupation of S. littoralis (Font; Cambria 10)*
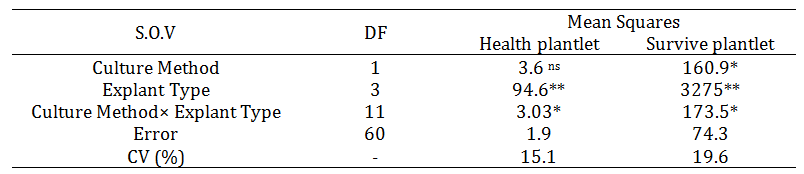
*, ** significant and 5 and 1 percent, respectively, ns; non-significant. The data in the table are the means ± SE. Different letters in the same column indicate significant differences at P < 0.05
*Tables should not have lines between their rows
*The top and bottom titles of the tables should be in the middle
*Note: The number of tables in each article should not exceed 5
4. Discussion
Text for this section (Font; Cambria 10 Regular)
5. Conclusions
Text for this section (Font; Cambria 10 Regular)
Abbreviation
Authors should write full words or phrases, abbreviated in the article text, in this section
Conflict of interest
All authors have to declare their conflict of interest.
Consent for publications
All authors have to write this sentence that they read and approved the final manuscript for publication.
Availability of data and material
The authors have to declare that they embedded all data in the manuscript.
Authors' contributions
All authors should write their part in designing the idea, doing, analyzing and writing the article.
Funding
Authors should mention the company, institution or organization that paid for the research
Ethics approval and consent to participate
The authors have to declare that they do not use human or animals in their research but if they used, they have to write name of the ethics committee that has approved the study of enter the code of research ethics (provided by the institution or university where the research was conducted) in the article.
Acknowledgement:
If necessary, the authors can thank people or institutions that have provided them with scientific or material assistance in doing and writing the article.
6. References
Maximum number of the references should be 35 titles.
All references should have doi
Arrange references as a simple list here by using the EndNote.
All references have to be hanging with 0.5 cm (The first line of each reference entry is aligned flush with the left margin and each subsequent line has a hanging indent of 0.5 cm) (Font; Cambria 10 Regular)
Note: The number of references must be a maximum of “35”, and on the other hand, all references must have a “doi”. No reference without “doi” in the article will be accepted.
Note: Authors are not allowed to cite their previous articles for more than 5 references
You can use the Endnote Style in below link:
https://www.cmbr-journal.com/data/cmbr/news/CMBR.ens
Examples:
References - In the text, number references in order of appearance using Arabic numerals [e.g. 1, 2, 3] in parentheses for citations. Multiple citations within a single set of brackets should be separated by commas. Where there are three or more sequential citations, they should be given as a range [2,3-7,15]. List the references at the end of the paper in numbered order. The list should contain at least fifteen references and should be arranged in the order of citation in text. References to articles must include: 1. name(s) and initials of author(s), if more than six (6) authors add an et al. after the sixth author.; 2. title of paper; 3. title of the journal in the standard manner (see Index Medicus/Pubmed); 4. publication year; 5. volume number; 6. first and last page numbers of the article (references to online articles should have the same structure and additionally the appropriate web address following page numbers).
Important: References numbers should be linked to reference list. Journal names should be in the most famous form (it doesn't matter whether it is abbreviate or full name) according to Cell. Mol. Biomed. Rep.. Authors are fully responsible for the accuracy of the references.
Journal
- Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15:59. doi: https://doi.org/10.3390/ijerph15010059
- Hall J, Soole K, Bentham R (2011) Hydrocarbon phytoremediation in the family Fabaceae--a review. Int J Phytoremediation 13:317-332. doi: https://doi.org/10.1080/15226514.2010.495143
Conference
- Abbasi-Moghadam J, Shahriari A, Fazeli-Nasab B (2017) Investigation of bacteria and fungi populations associated with airborne dust during ‘’wind of 120 days’’ blowing in the urban areas of Sistan plain. Paper presented at the 15th Iranian Soil Science Congress, Isfahan University of Technology, Isfahan, Iran, Congress COI: SSCI15, Article COI: SSCI15_687,
- Ali-Soufi M, Shahriari A, Shirmohammadi E, Fazeli-Nasab B (2017) Investigation of biological properties and microorganism identification in susceptible areas to wind erosion in Hamoun wetlands. Paper presented at the Congress on restoration policies and approaches of Hamoun international wetland Zabol
Book (book Chapter and book section)
- Fazeli-Nasab B, Sayyed RZ (2019) Plant Growth-Promoting Rhizobacteria and Salinity Stress: A Journey into the Soil. In: Sayyed RZ, Arora NK, Reddy MS (eds) Plant Growth Promoting Rhizobacteria for Sustainable Stress Management : Volume 1: Rhizobacteria in Abiotic Stress Management. Springer Singapore, Singapore, pp 21-34. doi: https://doi.org/10.1007/978-981-13-6536-2_2
- Rasouli H, Popović-Djordjević J, Sayyed RZ, Zarayneh S, Jafari M, Fazeli-Nasab B (2020) Nanoparticles: A New Threat to Crop Plants and Soil Rhizobia? In: Hayat S, Pichtel J, Faizan M, Fariduddin Q (eds) Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development. Springer International Publishing, Cham, pp 201-214. doi: https://doi.org/10.1007/978-3-030-33996-8_11
Web
- AMFEP (2015) Association of manufacturers and formulators of enzyme products. List of commercial enzymes. http://www.amfep.org/content/list-enzymes.
Appendix
Appendix 1 – Sample title
Descriptions text
Appendix 2 – Sample title
Descriptions text
Cell. Mol. Biomed. Rep. Article Types:
1 Review Article
These articles consist of Systematic Review, Meta-analysis, Systematic Reviews and Meta-analysis, Meta-Synthesis, Scoping Review, Literature Review, and Narrative Review. These articles may be up to 7000 words excluding abstract, tables, and references. Inclusion and exclusion criteria for studies should be described in a flow diagram. The specific type of study or analysis, intervention, population, exposure, outcomes or tests should be described for each data source or article. Authors are to clearly cover the following topics in the method section: search strategy and selection criteria, data extraction, quality assessment, and data analysis. A structured abstract is required that should include: Background; Methods and Analysis; Discussion; Registry and number (if the protocol of these articles had been registered in a registry system).
For these articles authors need to complete and include a PRISMA-P checklist (please find it from http://prisma-statement.org/prismastatement/Checklist.aspx) and upload as a supplementary file. Authors MUST ensure that all points are included and state page numbers where each item can be found.
- Study Protocol
IJHPM welcomes study protocols for any study design. We will not consider the manuscript if the data collection is complete. Protocols for studies that will require ethical approval, such as trials, will not be considered without receiving the approval. By publishing your study protocol at the IJHPM, you are not committed to submit subsequent reports of the study to IJHPM, although we do welcome such submissions.
Study protocols should be maximum 5000 words (excluding abstract, figures, tables, and references) and cover the following components:
- Title: this should include the specific study type, e.g. randomised controlled trial.
- Abstract: this should be structured with the following sections. Background; Methods and Analysis; Discussion; Registry and number (if the protocol had been registered in a registry system).
- Background: explain the rationale for the study and the gap in the literature it may fill.
- Methods and Analysis: provide a thorough description of the study design, including sample selection; interventions to be measured; the sample size calculation; procedures, measurements and analytical techniques; a data analysis plan.
- Discussion: discuss how the methods and statistics will meet the study aims.
We recommend registering the protocol in PROSPERO(https://www.crd.york.ac.uk/PROSPERO). This makes the review process for these articles faster as they have already passed a review process.
- Original Article
These articles must be of primary research, methodologically accurate, and relevant to international health policy and management. They should contain no more than 7000 words excluding structured abstract, tables, and references. Each manuscript should clearly state an objective or hypothesis; the design of study and methodology (including study setting, patients or participants, inclusion and exclusion criteria, sampling and data source); data analysis and interpretations; the main results of the study, discussing the results; addressing study limitations and the conclusion. For all original articles, a structured abstract is required (please see instructions for preparing Abstracts(https://www.ijhpm.com/journal/authors.note.php#Abstract)). In line with the Knowledge Translation movements, IJHPM has adopted an initiative by which all original articles are required to have Key Messages under two separate headings namely: Implications for Policy Makers and Implications for Public (please see instructions for preparing Key Messages(www.ijhpm.com/journal/authors.note#KeyMessages)). Only original articles require Key Messages.
You can submit your manuscript using the Sample Word Template provided here(https://www.cmbr-journal.com/contacts ).
- Short Communication
Short communications are short articles (mini original articles) that present original and important preliminary findings that do not warrant publication as a full-length article but are still worthy of publication. Short communications should have an un-structured abstract and should not be more than 4000 words including references and up to three tables or figures. The main text should be sub-divided into background, methods, results, and discussion, but should be written as concisely as possible. To maintain brevity, these articles do not need key messages.
- Commentary
These articles are invited on selected Cell. Mol. Biomed. Rep. publications mainly from leading scholars in the field. Authors of the lead article and editors decide whom to invite. These types of articles should not be more than 2000 words including maximum 2 figures or tables with an unstructured abstract.
- Correspondence
These articles are mainly written in response to published commentaries by authors whose articles have been subject to commentaries. These types of articles should not exceed 1000 words including maximum 1 table or figure, references and the main text. No abstract is required for these articles.
- Letter to Editor
We welcome short letters with topics of interest to the Cell. Mol. Biomed. Rep. readership. These letters should not exceed 700 words including only one table or figure, references and the main text. No abstract is required for these articles.
Please insert the type of your manuscript on top of the Title Page and Main Manuscript.

